Part 5: Autism and Health Issues Unpacked: Exploring the Intersection of Autism and Chronic Fatigue Syndrome
Written by Claire Eliza Sehinson, MS with Dr. Megan Anna Neff, PsyD. Originally posted on Neurodivergent Insights
This is a continuation from Part 4.
Insufficient Vitamin D, Essential Fatty Acids & Minerals
Nutritional Deficiencies in Autism and CFS
Autistic and CFS patients often exhibit nutritional deficiencies or sub-optimal levels of vital nutrients. Common deficiencies include vitamins (D, folate, B12, and C), long-chain omega fatty acids (EPA & DHA), and minerals (zinc, iron, magnesium).
Causes of Vitamin Insufficiencies:
These vitamin insufficiencies can have multiple causes:
Autoimmune Gut Conditions: For instance, pernicious anemia where the immune system attacks cells that produce a protein called ‘intrinsic factor’ that binds to vitamin B12 so it can be absorbed. Without intrinsic factor we see low B12 and this can lead to anemia.
Digestive Issues and Malabsorption: Clinical observations often reveal low stomach acid, crucial for mineral absorption such as zinc, magnesium and iron. Additionally, leaky gut can impede nutrient absorption due to damage to mucosal surface transporters.
Restrictive eating patterns: Sensory difficulties, ARFID or eating disorders can impact the range of foods eaten and this reduces the variety of nutrients the person is able to obtain from their diet
Geographical Factors: Vitamin D from sunlight is inadequate in many regions north of the equator. An easy way to tell is the vitamin D shadow rule.
Genetic Factors: Variations like MTHFR can affect folate activation.
Mineral Status and Vitamin D: Low vitamin D levels can impact calcium and magnesium absorption.
Modern Agriculture: Modern farming methods and soil depletion reduces the nutrient density of foods, particularly selenium and magnesium.
Essential Fatty Acids: Omegas 3,6,9 are much lower in the modern diet with higher processed foods and lower whole-foods. Fat malabsorption disorders can hinder fat-soluble vitamin absorption (i.e. vitamin D or A) even if the person is eating sufficient amounts. Viral infections can additionally impair our ability to make long-chain fatty acids.
Vegan diets: Vegan diets are typically low in iron and B12 and may require supplementation
Heavy menstruation: Heavy menstration can results in iron loss.
Chronic infections: Bacteria and fungal infections utilize iron for their own metabolism
Chronic stress: Stress can lower stomach acid, reroute nutrients like zinc and vitamin C to support the stress response, and detract from other bodily functions.
Dietary factors: High sugar consumption can deplete vitamin C, while diuretics like caffeine or alcohol increase the loss of B vitamins, electrolytes, and minerals.
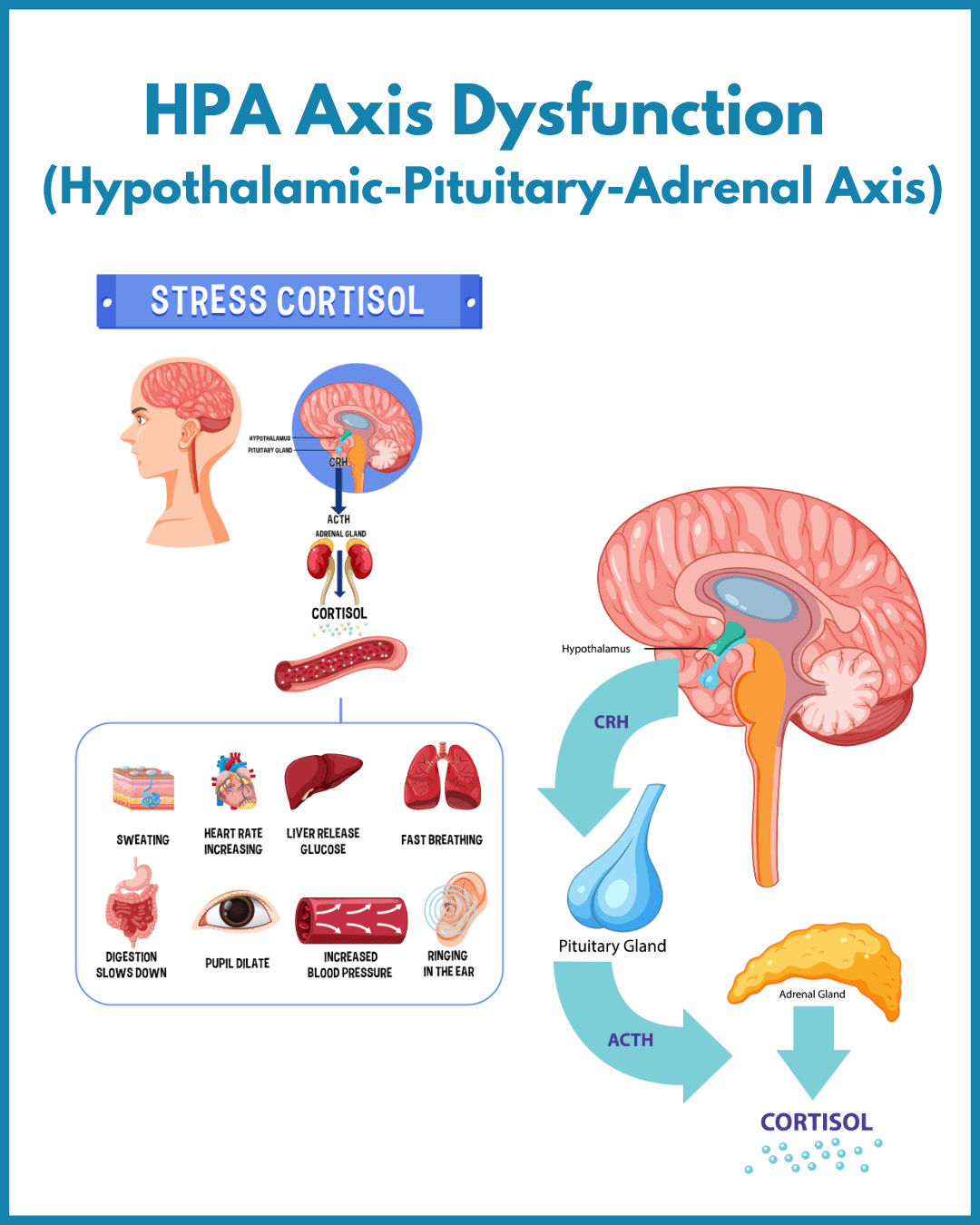
Hypothalamic-Pituitary-Adrenal (HPA) Axis Dysfunction
The HPA-Axis refers to the complex neuro-endocrine feedback loop between the hypothalamus and pituitary (in the brain) and the adrenal glands that sit above our kidneys.This system orchestrates the body's response to stress.
Stress Response Mechanism:
During physiological and psychological stress “fight-flight-freeze”, the hypothalamus releases corticotropin-releasing hormone, stimulating the adrenal cortex to release cortisol and DHEA. These hormones prepare the body for “survival” by increasing energy availability to our muscles and brain, whilst shutting down non-essential processes like digestion and the immune system.
The adrenal glands also produce catecholamines (adrenaline, noradrenaline, and dopamine), which drive the “reflex reactions” in acute stress situations.
Short periods of adrenal stimulation favors survival in immediate danger or threat. However, prolonged and persistent stimulation of the stress response is demonstrated to play a role in the development of a multitude of metabolic disorders as well as CFS, cognitive decline, burnout and the majority of chronic illnesses (Tomas et al., 2013).
HPA Axis Dysfunction in Chronic Fatigue:
Research consistently describes a state of HPA axis dysfunction in people with chronic fatigue and this shows up as:
A disruption of circadian rhythm - that can affect waking energy and getting to sleep
Reduced cortisol output in the long run because cortisol receptors in the brain become overly sensitized to cortisol and reduce cortisol output (“negative feedback loop”) - clients with this pattern tend to describe and loss of resilience and a “tired-but-wired” feeling like they are running on adrenaline
In particular a blunted morning surge of cortisol - clinically this looks like a person who lacks the “get up and go” and feels completely exhausted
*HPA axis dysfunction is also more prevalent in females
Autistic children and adults have highly sensitive nervous systems and many types of social and sensory input can be experienced as overwhelming, unpleasant or even threatening. Our nervous system can go into fight-flight-freeze with alarming regularity to seemingly “nothing”. This chronic stress activation has been demonstrated to result in HPA axis dysfunction with consequences such as poor immune function and inflammation (Makris et al., 2021).
Chronic Hypervigilance “Limbic Kindling”
In this context, the nervous system's response to perceived threats can become exaggerated over time, a phenomenon known as “limbic kindling.” The limbic system (part of our brain responsible for survival, fear, emotions and memory) can become “hard-wired” to react to perceived threats more easily, even if the original threat is no-longer there. This means that the HPA axis is altered in a way that less stimulus is needed to trigger a state of hypervigilence (Gelhorn, 1970). This heightened state of alertness can further exacerbate HPA axis dysfunction and its associated health impacts.**
Hypersensitization in CFS/ME
This heightened sensitivity to stimuli, whether chemical, infectious, or psychological, has been observed in CFS/ME patients (Gupta, 2002). Limbic kindling can significantly affect the health of individuals with CFS, leading to a variety of issues. As outlined by Jason et al., 2011, limbic kindling in CFS can lead to:
Imbalances in the immune system.
Increased allergic reactions.
More frequent chronic infections.
Reduced brain volume.
Lower levels of GABA and acetylcholine, crucial neurotransmitters for the parasympathetic nervous system, which plays a key role in relaxation and recovery.
Neuroinflammation in ME/CFS and Autism
Neuroinflammation in ME/CFS:
Many of the core symptoms of ME/CFS, such as brain fog, fatigue, pain, and sensory sensitivities, are linked to neuroinflammation. The brain's immune cells, known as microglia, play a central role in this process. When microglia detect that something is wrong, like presence of pathogens, low oxygen levels, or inflammation in other body parts (like the gut), they become activated. This activation leads to the production of inflammatory and excitatory chemicals, such as glutamate, heightening nerve signaling in the brain. This can manifest as increased sensitivity and difficulty concentrating. Neuroimaging studies of CFS patients, such as Nakatomi et al. (2014), have identified neuroinflammation in critical brain areas related to pain processing, brain stem function (including the vagus nerve), and autonomic processing (which regulates heart rate, respiratory rate, and reflexes).
Neuroinflammation in Autism
Similar patterns of neuroinflammation and microglial activation have been consistently observed in Autistic children (Tsilioni et al., 2019). Autistic research often focuses on the connection between neuroinflammation and social behaviors, communication difficulties, and the atypical wiring of the limbic system. However, insights from neuroinflammation research in CFS and other chronic illnesses offer valuable perspectives. By understanding and addressing neuroinflammation, it may be possible to alleviate symptoms like pain, sensory overload, and hypervigilance, leading to reduced distress behaviors in Autistic individuals.
Understanding Low Vagal Tone
The Vagus Nerve: A Key Component in the Body-Brain Communication
The vagus nerve, or the 10th cranial nerve, originates from the brainstem and connects to most major organs. It's aptly named “Vagus” (Latin for 'wandering')as it connects to all of your major organs. The communication via the vagus nerve is bi-directional, allowing signals to travel both from the brain to the body and vice versa. Interestingly, the majority (about 80%) of this neural communication is from the body to the brain. The vagus nerve is the main nerve that innervates the gut – and is what we refer to when we talk about the gut-brain-axis.
Role of the Vagus Nerve
As the dominant nerve of the parasympathetic nervous system, the vagus nerve plays a role various conditions, including depression, PTSD, immune dysfunction, autoimmunity, pain, epilepsy, cardiovascular disease, cognitive decline, autism, ADHD, fibromyalgia, tinnitus, sleep issues, aging, and more.
Vagal Tone
The vagus can have high or low “tone” describing the fitness of this nerve. Good vagal tone is associated with a range of positive health outcomes, including a longer lifespan, reduced stress and pain, and improved mood. Conversely, poor vagal tone is linked with stress-related chronic illnesses, digestive issues (like constipation), sleep disorders, heart disease, and depression (Bonas et al., 2016; Bonaz et al., 2017).
Low Vagal Tone in Autism and CFS
Poor vagal tone has long been observed in Autistic and CFS populations, indicating less flexibility in their nervous systems. For Autistic people this might result in stress easily induced by social encounters, sensory input, routine disruption, unexpected changes, and difficulties with emotional regulation.
With CFS, as vagal tone declines over a lifetime people tend to find their resilience to any environmental stimuli or interpersonal triggers decrease and they become more reactive to everything. These experiences echo the sensory overload and emotional dysregulation often experienced by Autistic people from birth and low vagal tone is one of the overlapping factors.
Electrical Stimulation for Vagal Tone Improvement
Encouragingly, there are many ways to improve vagal tone. Through lifetime modifications, limbic retraining and vagal nerve stimulators. Studies exploring electrical stimulation, like using a TENS machine, have shown promise in improving symptoms of chronic fatigue and fibromyalgia. For instance, Traianos et al. (2021) demonstrated symptom improvement in CFS and fibromyalgia patients through non-invasive electrical stimulation.
Food and Chemical Sensitivities in Autism and CFS
The majority of Autistic individuals and CFS patients report sensitivities to multiple foods and chemicals. They can produce an array of bewildering and worrying symptoms affecting the whole body for prolonged periods of time - so it is important to distinguish what they are in order for them to be managed properly.
Clinically these reactions can be broken down into types.
Immunological Reactions: These include immune-based reactions like allergies, intolerances, or mast-cell activation. Mediated by antibodies (IgE, IgG), inflammatory cascades, or histamine levels, these reactions often have common triggers due to overlapping proteins. For example, birch pollens and related fruits like apples, almonds, peaches, and cherries. Leaky gut syndrome can also lead to large food proteins entering the bloodstream, linking to autoimmune gut disorders (such as Celiac or Crohn’s disease) or atopic conditions (eczema, asthma, hayfever).
Interoceptive Sensitivities: These involve sensitization to the characteristics of food or chemicals, such as texture, taste, odor, temperature, eating environment, or associations with specific memories (e.g., choking, vomiting, food poisoning). This type often presents as IBS, with stomach upsets being a common manifestation of stress and overload. The enteric nervous system (nervous system that innervates the entire digestive tract) plays a significant role here. Antibody tests typically show no abnormalities, and the condition is not linked to inflammation. Unlike immunological reactions, interoceptive sensitivities vary widely and relate to individual sensory preferences and aversions. Addressing these sensitivities, especially in conditions like ARFID (avoidant restrictive food intake disorder) and non-inflammatory IBS, requires a nuanced understanding of interoception and fostering a safe environment around food in a Neurodivergent-affirmative way.
Toxicity and Its Impact
Understanding Toxicity
The concept of toxicity in mainstream research lacks a clear consensus. Toxicological studies looking at safety upper limits of chemicals focus on how much of a single toxin is required to cause harm in living organisms. In reality, toxin exposure does not work like this. We have low level exposure to thousands of toxins simultaneously (at levels that have been considered “safe”) and this multiplicity in exposure can overwhelm our capacity to excrete them.
A landmark investigation by the Environmental Working Group looked at the body burden of chemicals, pollutants and pesticides in the umbilical cord blood of newborn babies and found an average of 200 chemicals, including 217 toxic to the brain and nervous system or carcinogens. This combined effect of multiple environmental pollutants has not been studied in human trials.
Clinical Observations and Functional Medicine Approach
Clinically, we see how accumulated toxic exposures over a lifetime can surpass the body's detoxification capabilities. These toxins, deposited in tissues, can disrupt biological processes and lead to symptoms of toxicity. In functional medicine, the focus is on the intersection of genetic vulnerability and lifestyle-based susceptibility to toxins, devising strategies to facilitate their safe excretion.
Clinically we see how accumulated toxic exposures over a lifetime eventually outweigh the person’s capacity to detoxify/remove these from the body. These circulating toxins get deposited into our tissues and can disrupt normal biological processes resulting in widespread symptoms of toxicity. Therefore in functional medicine, the focus is often on the intersection between genetic vulnerability and lifestyle-based susceptibility of being exposed to certain types of toxins and working out how to facilitate the excretion of these toxins safely through nutritional, lifestyle or supplemental strategies.
There have been some key toxins associated with Autism and CFS, including:
Toxic metals: nickel, lead, mercury (Wojcik, 2006; Blazewicz et al., 2023; Yakob, 2006)
Organophosphates: Such as insecticides, DEET, glyphosate (Dunstan et al., 2005; Sagiv et al., 2018).
Biotoxins: Mold/mycotoxins (Brewer et al., 2013), viral and bacterial toxins (Morris et al., 2015).
Vulnerability to Toxicity
There are several factors influencing why some people are more vulnerable to toxicity and develop complex chronic illness, whilst others (including their partners and family members) may be exposed to the same toxins but do not become ill or symptomatic. Some factors include:
Genetic variants affecting detoxification capacity: Certain genetic variants can significantly influence the body's ability to detoxify. For example, the GSTM1 gene typically encodes a protein essential for removing environmental pollutants and heavy metals from our cells. However, in some individuals, this gene may be reduced in function or deleted entirely – a condition known as GSTM1 deletion. This genetic alteration can lead to an accumulation of toxins in the body, a phenomenon known as "bioaccumulation." This build-up of toxins due to impaired detoxification has been documented in studies by Nakanishi et al. (2022) and Aronica et al. (2022).
Nutritional deficiencies can exacerbate metal toxicity: Nutritional deficiencies, particularly in essential minerals like calcium, selenium, and zinc, can inadvertently increase the body's absorption of toxic metals such as lead, mercury, and nickel. This happens because these toxic metals are structurally similar to essential minerals and compete for the same absorption pathways or transporters in the body.
When essential mineral levels are low, the body may mistakenly absorb more of these toxic metals. For example, in the absence of adequate calcium, lead can be deposited into bones, a site where calcium is normally found. In these bones, lead can disrupt normal functions, leading to various health issues. Similarly, deficiencies in selenium and zinc can lead to increased accumulation of other toxic metals in organs where these essential minerals are usually utilized.
Therefore, one effective strategy to reduce the risk of metal toxicity is to maintain adequate levels of essential minerals through a balanced diet or supplementation as needed. This approach helps ensure that the body preferentially absorbs and utilizes these beneficial minerals, reducing the likelihood of toxic metal accumulation.
The Impact of Digestive Function on Detoxification:Poor digestive function, particularly constipation, plays a significant role in the body's detoxification process. The gut is a key player in eliminating toxins, largely due to its role in processing bile. Here's how it works:
Bile and Toxin Elimination: The liver deposits most of the body's toxins into bile. This bile, secreted by the gallbladder, then travels into the digestive tract.
Fiber's Role: Once in the digestive tract, bile binds to dietary fiber. This binding is crucial because it allows toxins to be carried out of the body with the stool, effectively completing the detoxification process.
Constipation and Toxin Reabsorption: However, if constipation occurs, this process can be disrupted. Severe constipation can lead to a delay in excreting bile-bound toxins. When this happens, there's a risk that these toxins can be reabsorbed back into the bloodstream instead of being excreted. This phenomenon, known as “entero-hepatic recirculation,” can lead to a buildup of toxins in the body.
Chronic Inflammatory Response Syndrome (CIRS): Chronic Inflammatory Response Syndrome (CIRS) is a condition identified in a significant portion of the population with chronic fatigue-like illnesses. A large DNA analyses of over 12,000 patients with chronic fatigue-like illnesses found that 24% of the population are unable to make antibodies to certain types of biotoxins/pathogens - such as mycotoxins (Shoemaker., 2014). The implications of this are significant. These individuals are unable to mount an effective immune response to neutralize and eliminate these toxins. Consequently, they tend to accumulate toxins in their bodies and experience exaggerated chronic inflammatory responses, even to minimal exposures. This results in a wide constellation of symptoms that most CFS and Autistic people will find familiar
Interestingly, this inability to produce specific antibodies is often linked to mutations in the HLA DR gene set. These genes have associations with numerous autoimmune conditions, suggesting a genetic predisposition to CIRS in certain individuals.
Further Reading
For a functional approach to understanding and managing toxicity see Dr. Jill Crista's work on mold-related illness and Chronic Inflammatory Response Syndrome:
Toxic by Neil Nathan MD
Dr Jill Crista for mold related illness and CIRS (Chronic Inflammatory Response Syndrome)
Pyroluria
Overview and Prevalence
Pyroluria is a metabolic condition that can be either genetic or acquired, affecting up to 10% of the population. Notably, higher incidences of Pyroluria are seen in conditions like schizophrenia, OCD, social anxiety, substance misuse, the autistic spectrum, and CFS (McGinnis et al., 2008).
Discovery and Mechanism
Discovered in the 1950s by Dr. Abraham Hoffer and Dr. Carl Pfeiffer, Pyroluria involves the overproduction of kryptopyrroles, by-products of hemoglobin synthesis. Hemoglobin carries oxygen in our red blood cells, but kryptopyrroles themselves don't serve any biological purpose and are typically harmless in small amounts. However, when produced excessively, they bind to zinc and vitamin B6, rendering these nutrients unavailable to tissues and cells. This binding leads to a loss of zinc and B6 through urine, a process that can be measured to diagnose Pyroluria.
Symptoms, Impacts And Clinical Approach
This depletion leads to chronic deficiency of zinc and B6 with the emergence of a myriad of wide-ranging symptoms. Zinc is crucial for over 300 bodily functions, especially in brain health, immune function, and stress response. Similarly, B6 is essential for neurotransmitter and hormone balance. Patients often describe a severe feeling of inner tension or poor stress tolerance.
It is believed that emotional trauma or stress can be a trigger to this process, but because zinc and B6 are such important nutrients for a healthy stress response, it then becomes more difficult for that person to bounce back from the episode and the vicious cycle continues. As a practitioner, pyroluria is something we try to identify early on because it is “treatable” with zinc/B6 repletion.
EMF Sensitivity: Understanding and Managing Exposure
What are Electromagnetic Fields (EMFs)?
EMFs are a combination of two naturally occurring invisible fields: magnetic (like the force between two magnets) and electrical (like the current flowing through a wire). In our bodies, our brain and nervous system use electrical currents to transmit information. EMFs occur where these electrical and magnetic forces combine.
Impact of Human-Made EMFs
Prolonged exposure to human-made EMFs, such as those from WiFi, can lead to hypersensitivities, particularly in vulnerable individuals. While research on safe exposure levels is ongoing, clinical observations often report EMF sensitivities in people with severe CFS and autism. Symptoms may diminish when exposure is minimized. The main biological effects and impacts on human health are outlined in the diagram below (Pall, 2018)
Biological Effects and Health Impacts
Some key mechanisms explaining symptoms seen in CFS/autism include:
Activation of Voltage-Gated Ion Channels: EMFs can activate "voltage-gated ion channels" on cell walls, particularly those of neurons. These channels function similarly to an electronic key card operating a door. When EMFs trigger these channels, they open, allowing calcium ions to flood into the cell. Calcium plays a crucial role as a signaling molecule inside our cells. For instance, in brain cells, this influx of calcium can initiate the release of neurotransmitters. In immune cells like mast cells, it can lead to the release of histamine and other chemicals. Consequently, EMF exposure can heighten various "sensitivities" due to these cellular responses.
Disruption of Melatonin Production: EMF disrupts melatonin production by the pineal gland and this has an obvious impact on sleep, but also inflammation (as melatonin has a huge anti-inflammatory impact on our cells) and antioxidant levels (again melatonin is one of our biggest antioxidant hormones that are protective to cell structures and our DNA).
Reactivation of Viruses: EMFs have been demonstrated to reactivate Epstein Barr Virus with as little as 50hz of EMF for 72 hours (Grimaldi et al., 1997). 50hz is considered low level exposure and is what most of us are exposed to daily (everyday electronic devices and power transmission lines).
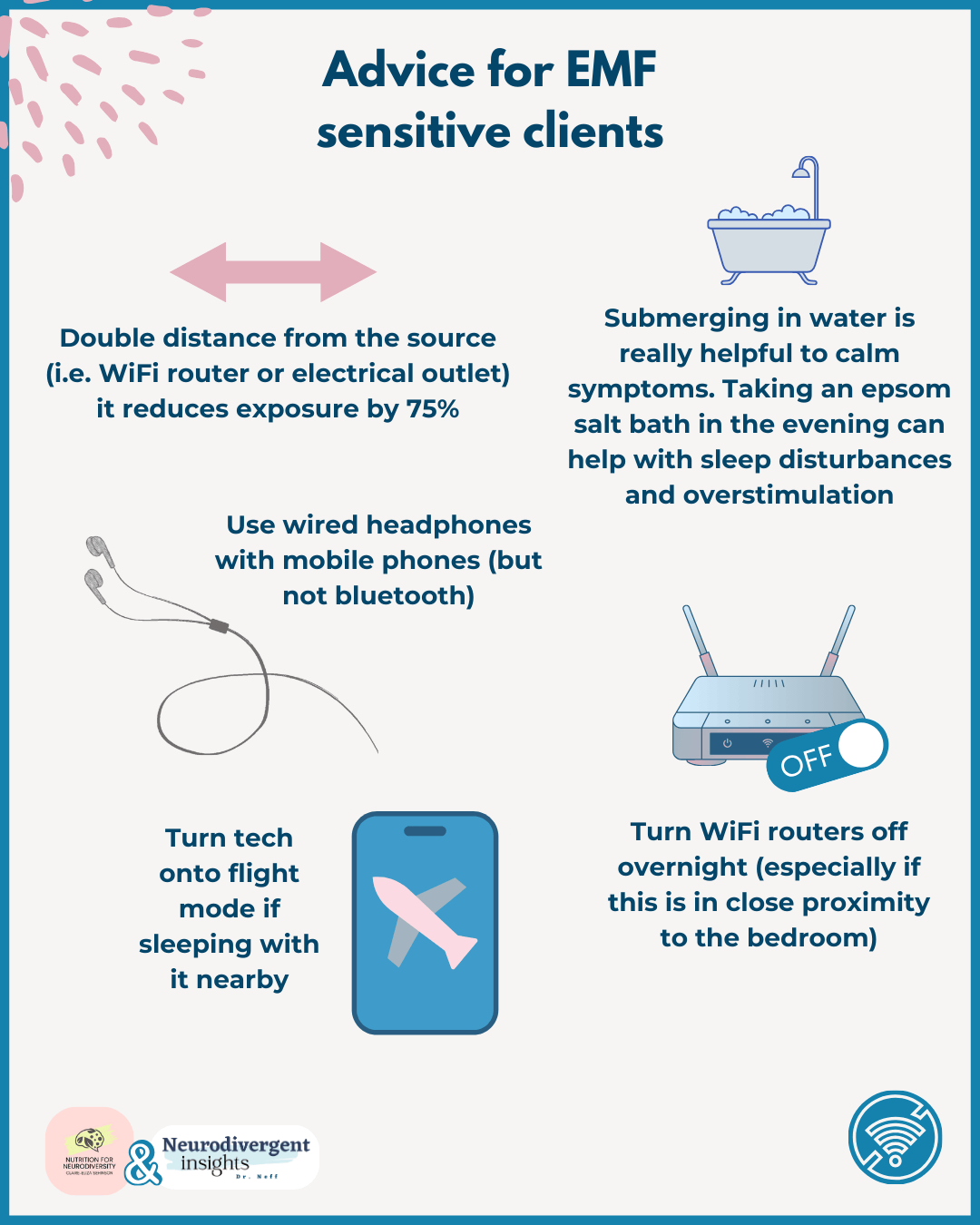
Practical Tips for Managing EMF Sensitivity
For clients sensitive to EMFs, here are some top tips:
Double distance from the source (i.e. WiFi router or electrical outlet) it reduces exposure by 75%
Submerging in water is really helpful to calm symptoms. Taking an epsom salt bath in the evening can help with sleep disturbances and overstimulation
Use wired headphones with mobile phones (but not bluetooth)
Turn WiFi routers off overnight (especially if this is in close proximity to the bedroom)
Turn tech onto flight mode if sleeping with it nearby
Pain and Fibromyalgia in Neurodivergent Adults
Altered Pain Perception in Autism and ADHD
Autistic people and ADHDers often experience significant alterations in pain perception. They are more likely than neurotypical individuals to suffer from increased pain sensitivity, chronic widespread pain, and pain-related anxiety (Failla et al., 2020). One study found that 80% of adults with ADHD experience chronic widespread pain, compared to just 17% of neurotypical adults (Stray et al., 2013).
Understanding Fibromyalgia
Fibromyalgia is a chronic condition primarily characterized by widespread pain in connective tissues and muscles. Patients often experience a constellation of symptoms, including profound fatigue, anxiety, sleep disturbances, and cognitive challenges. While the precise cause of Fibromyalgia remains elusive, it is understood as an abnormality in how the brain processes pain signals. This abnormal processing can stem from various factors, including emotional trauma, genetic predispositions, or neuroinflammation. A key feature of Fibromyalgia is a heightened sensitivity to stimuli, leading to intensified pain experiences.
Fibromyalgia and Chronic Fatigue Syndrome (CFS)
There is a notable overlap between Fibromyalgia and Chronic Fatigue Syndrome (CFS). Research indicates that 30-55% of individuals diagnosed with CFS also meet the criteria for Fibromyalgia. Intriguingly, this comorbidity rate can rise to as high as 70% among patients with Fibromyalgia, suggesting a strong clinical and possibly pathophysiological connection between these two conditions (Afari and Buchwald, 2003).
Fibromyalgia and Neurodivergence
Recent research indicates a significant overlap between fibromyalgia and neurodivergence. For instance, a study screening 123 adults with fiibromyalgia for ADHD found that approximately 45% met the criteria for ADHD (van Rensburg, 2018). Research into pain mechanisms in neurodivergent individuals often points to centralized sensitization and neuroinflammation as underlying factors, paralleling findings in fibromyalgia studies.
Additionally, in clinical practice and research into Autistic children, it's been observed that parents (especially mothers) of Autistic children frequently have diagnoses of CFS/ME or Fibromyalgia. Where testing was available, there were many shared heritable factors such as mitochondrial dysfunction (passed down the maternal lineage), shared genomics in inflammatory, histamine or methylation pathways and shared immune dysfunction. Given that autism has a 90% heritability rate it is highly likely that these conditions overlap. Considering the high heritability of autism, there's a strong likelihood of overlap between these conditions.
It is our belief that a subset of individuals with CFS/ME and Fibromyalgia might represent a “lost generation” of undiagnosed Autistic and ADHD individuals, suggesting a deeper interconnectedness between these conditions and neurodivergence.
Conclusion
This article has delved into a range of complex and intertwined health conditions, with a special focus on the connections between autism, chronic fatigue syndrome (CFS), and other conditions commonly experienced alongside them.
We've examined key themes such as the impact of sleep disturbances, gut health issues, methylation and genetic factors, as well as the role of the HPA axis and vagal tone. The challenges posed by environmental factors like EMF sensitivity and mycotoxin exposure were also explored. Additionally, we delved into how dysautonomia, limbic kindling, and nutritional deficiencies crucially influence the manifestation and experience of these health conditions.
An important aspect that emerged is the concept of diagnostic overshadowing, where the presence of a well-known condition like autism or CFS can lead to the overlooking or misattributing of symptoms related to other co-occurring conditions. This highlights the need for a more comprehensive, nuanced approach to diagnosis and treatment.
This exploration underscores the necessity of recognizing individual differences and tailoring interventions to address the unique constellation of symptoms and underlying causes. As research continues to evolve and our understanding deepens, it is our hope that this article serves as a resource to shed light on these complex conditions and offer guidance for those navigating them, whether as patients, caregivers, or healthcare professionals. By embracing a comprehensive, empathetic approach to healthcare, we can better support those facing these often challenging and interwoven health journeys.
Further Learning
All The Things: Dr. Mel Houser’s website and work does a great job of highlighting the intersection of neurodivergence and “all the things.” You can listen to them on the podcast, Divergent Conversations here.
References
Aaron RV, Fisher EA, de la Vega R, Lumley MA, Palermo TM. Alexithymia in individuals with chronic pain and its relation to pain intensity, physical interference, depression, and anxiety: a systematic review and meta-analysis. Pain. 2019 May;160(5):994-1006. doi: 10.1097/j.pain.0000000000001487. PMID: 31009416; PMCID: PMC6688175.
Afari, N., & Buchwald, D. (2003). Chronic fatigue syndrome: a review. American Journal of Psychiatry, 160(2), 221-236.
Aronica L, Ordovas JM, Volkov A, Lamb JJ, Stone PM, Minich D, Leary M, Class M, Metti D, Larson IA, Contractor N, Eck B, Bland JS. Genetic Biomarkers of Metabolic Detoxification for Personalized Lifestyle Medicine. Nutrients. 2022 Feb 11;14(4):768. doi: 10.3390/nu14040768. PMID: 35215417; PMCID: PMC8876337.
Acheson ED. The clinical syndrome variously called benign myalgic encephalomyelitis, Iceland disease and epidemic neuromyasthenia. Am J Med. 1959 Apr;26(4):569-95. doi: 10.1016/0002-9343(59)90280-3. PMID: 13637100.
Atchison CJ, Davies B, Cooper E, Lound A, Whitaker M, Hampshire A, Azor A, Donnelly CA, Chadeau-Hyam M, Cooke GS, Ward H, Elliott P. Long-term health impacts of COVID-19 among 242,712 adults in England. Nat Commun. 2023 Oct 24;14(1):6588. doi: 10.1038/s41467-023-41879-2. PMID: 37875536; PMCID: PMC10598213.
Bennett JA, Germani T, Haqq AM, Zwaigenbaum L. Autism spectrum disorder in Prader-Willi syndrome: A systematic review. Am J Med Genet A. 2015 Dec;167A(12):2936-44. doi: 10.1002/ajmg.a.37286. Epub 2015 Aug 29. PMID: 26331980.
Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-49. doi: 10.1515/reveh-2015-0026. PMID: 26613325.
Booth NE, Myhill S, McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Int J Clin Exp Med. 2012;5(3):208-20. Epub 2012 Jun 15. PMID: 22837795; PMCID: PMC3403556.
Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016 Oct 15;594(20):5781-5790. doi: 10.1113/JP271539. Epub 2016 May 1. PMID: 27059884; PMCID: PMC5063949.
Bonaz B, Lane RD, Oshinsky ML, Kenny PJ, Sinha R, Mayer EA, Critchley HD. Diseases, Disorders, and Comorbidities of Interoception. Trends Neurosci. 2021 Jan;44(1):39-51. doi: 10.1016/j.tins.2020.09.009. PMID: 33378656.
Bonaz B, Sinniger V, Pellissier S. The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Front Immunol. 2017 Nov 2;8:1452. doi: 10.3389/fimmu.2017.01452. PMID: 29163522; PMCID: PMC5673632.
Brewer JH, Thrasher JD, Straus DC, Madison RA, Hooper D. Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins (Basel). 2013 Apr 11;5(4):605-17. doi: 10.3390/toxins5040605. PMID: 23580077; PMCID: PMC3705282.
Buckley L, MacHale SM, Cavanagh JT, Sharpe M, Deary IJ, Lawrie SM. Personality dimensions in chronic fatigue syndrome and depression. J Psychosom Res. 1999 Apr;46(4):395-400. doi: 10.1016/s0022-3999(98)00120-2. PMID: 10340240.
Chao LL, Kriger S, Buckley S, Ng P, Mueller SG. Effects of low-level sarin and cyclosarin exposure on hippocampal subfields in Gulf War Veterans. Neurotoxicology. 2014 Sep;44:263-9. doi: 10.1016/j.neuro.2014.07.003. Epub 2014 Jul 21. PMID: 25058901; PMCID: PMC5464327.
Chu, L., Valencia, I. J., Garvert, D. W., & Montoya, J. G. (2019). Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Frontiers in pediatrics, 7, 12.
Crompton, C. J., Sharp, M., Axbey, H., Fletcher-Watson, S., Flynn, E. G., & Ropar, D. (2020). Neurotype-matching, but not being autistic, influences self and observer ratings of interpersonal rapport. Frontiers in Psychology, 11, 2961.
Csecs JLL, Iodice V, Rae CL, Brooke A, Simmons R, Quadt L, Savage GK, Dowell NG, Prowse F, Themelis K, Mathias CJ, Critchley HD, Eccles JA. Joint Hypermobility Links Neurodivergence to Dysautonomia and Pain. Front Psychiatry. 2022 Feb 2;12:786916. doi: 10.3389/fpsyt.2021.786916. PMID: 35185636; PMCID: PMC8847158.
Dancey CP, Friend J. Symptoms, impairment and illness intrusiveness--their relationship with depression in women with CFS/ME. Psychol Health. 2008;23(8):983-99. doi: 10.1080/08870440701619957. PMID: 25160923.
Daniels, J., Brigden, A., & Kacorova, A. (2017). Anxiety and depression in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): examining the incidence of health anxiety in CFS/ME. Psychology and Psychotherapy: Theory, Research and Practice, 90(3), 502-509.
Dargenio VN, Dargenio C, Castellaneta S, De Giacomo A, Laguardia M, Schettini F, Francavilla R, Cristofori F. Intestinal Barrier Dysfunction and Microbiota-Gut-Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder. Nutrients. 2023 Mar 27;15(7):1620. doi: 10.3390/nu15071620. PMID: 37049461; PMCID: PMC10096948.
de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010 Oct;51(4):418-24. doi: 10.1097/MPG.0b013e3181dcc4a5. PMID: 20683204.
Dong H, Zhang X, Qian Y. Mast cells and neuroinflammation. Med Sci Monit Basic Res. 2014 Dec 21;20:200-6. doi: 10.12659/MSMBR.893093. PMID: 25529562; PMCID: PMC4282993.
Eisenlohr-Moul T, Divine M, Schmalenberger K, Murphy L, Buchert B, Wagner-Schuman M, Kania A, Raja S, Miller AB, Barone J, Ross J. Prevalence of lifetime self-injurious thoughts and behaviors in a global sample of 599 patients reporting prospectively confirmed diagnosis with premenstrual dysphoric disorder. BMC Psychiatry. 2022 Mar 19;22(1):199. doi: 10.1186/s12888-022-03851-0. PMID: 35303811; PMCID: PMC8933886.
Giloteaux, L., Goodrich, J.K., Walters, W.A. et al. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 4, 30 (2016).
Gotts ZM, Deary V, Newton J, Van der Dussen D, De Roy P, Ellis JG. Are there sleep-specific phenotypes in patients with chronic fatigue syndrome? A cross-sectional polysomnography analysis. BMJ Open. 2013 Jun 20;3(6):e002999. doi: 10.1136/bmjopen-2013-002999. PMID: 23794547; PMCID: PMC3669720.
Groenman AP, Torenvliet C, Radhoe TA, Agelink van Rentergem JA, Geurts HM. Menstruation and menopause in autistic adults: Periods of importance? Autism. 2022 Aug;26(6):1563-1572. doi: 10.1177/13623613211059721. Epub 2021 Nov 26. PMID: 34825585; PMCID: PMC9344571.
Engineer CT, Hays SA, Kilgard MP. Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders. J Neurodev Disord. 2017 Jul 4;9:20. doi: 10.1186/s11689-017-9203-z. PMID: 28690686; PMCID: PMC5496407.
Espinosa P, Urra JM. Decreased Expression of the CD57 Molecule in T Lymphocytes of Patients with Chronic Fatigue Syndrome. Mol Neurobiol. 2019 Sep;56(9):6581-6585. doi: 10.1007/s12035-019-1549-7. Epub 2019 Mar 21. PMID: 30895436.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74. doi: 10.1016/j.cell.2011.02.013. PMID: 21376230.
Heim C, Wagner D, Maloney E, Papanicolaou DA, Solomon L, Jones JF, Unger ER, Reeves WC. Early adverse experience and risk for chronic fatigue syndrome: results from a population-based study. Arch Gen Psychiatry. 2006 Nov;63(11):1258-66. doi: 10.1001/archpsyc.63.11.1258. PMID: 17088506.
Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry. 2009 Jan;66(1):72-80. doi: 10.1001/archgenpsychiatry.2008.508. PMID: 19124690.
Kojima, M. Alexithymia as a prognostic risk factor for health problems: a brief review of epidemiological studies. BioPsychoSocial Med 6, 21 (2012). https://doi.org/10.1186/1751-0759-6-21
Kong, X., Zhu, J., Tian, R., Liu, S., Sherman, H. T., Zhang, X., ... & Wan, G. (2020). Early screening and risk factors of autism spectrum disorder in a large cohort of Chinese patients with Prader-Willi syndrome. Frontiers in psychiatry, 11, 594934.
Kossewska J, Bierlit K, Trajkovski V. Personality, Anxiety, and Stress in Patients with Small Intestine Bacterial Overgrowth Syndrome. The Polish Preliminary Study. Int J Environ Res Public Health. 2022 Dec 21;20(1):93. doi: 10.3390/ijerph20010093. PMID: 36612414; PMCID: PMC9819554.
Lee M, Krishnamurthy J, Susi A, Sullivan C, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Nylund CM. Association of Autism Spectrum Disorders and Inflammatory Bowel Disease. J Autism Dev Disord. 2018 May;48(5):1523-1529. doi: 10.1007/s10803-017-3409-5. PMID: 29170940.
Li Y, Qiu S, Shi J, Guo Y, Li Z, Cheng Y, Liu Y. Association between MTHFR C677T/A1298C and susceptibility to autism spectrum disorders: a meta-analysis. BMC Pediatr. 2020 Sep 24;20(1):449. doi: 10.1186/s12887-020-02330-3. PMID: 32972375; PMCID: PMC7517654.
Liu CH, Yang MH, Zhang GZ, Wang XX, Li B, Li M, Woelfer M, Walter M, Wang L. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020 Feb 12;17(1):54. doi: 10.1186/s12974-020-01732-5. PMID: 32050990; PMCID: PMC7017619.
Lievesley K, Rimes KA, Chalder T. A review of the predisposing, precipitating and perpetuating factors in Chronic Fatigue Syndrome in children and adolescents. Clin Psychol Rev. 2014 Apr;34(3):233-48. doi: 10.1016/j.cpr.2014.02.002. Epub 2014 Mar 1. PMID: 24632047.
Maes M, Mihaylova I, De Ruyter M. Lower serum zinc in Chronic Fatigue Syndrome (CFS): relationships to immune dysfunctions and relevance for the oxidative stress status in CFS. J Affect Disord. 2006 Feb;90(2-3):141-7. doi: 10.1016/j.jad.2005.11.002. Epub 2005 Dec 9. PMID: 16338007.
Maes M, Mihaylova I, Leunis JC. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord. 2007 Apr;99(1-3):237-40. doi: 10.1016/j.jad.2006.08.021. Epub 2006 Sep 27. PMID: 17007934.
Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. 2008 Dec;29(6):902-10. PMID: 19112401.
Magnusson AE, Nias DK, White PD. Is perfectionism associated with fatigue? J Psychosom Res. 1996 Oct;41(4):377-83. doi: 10.1016/s0022-3999(96)00189-4. PMID: 8971668.
Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007 May;85(5):1185-96. doi: 10.1093/ajcn/85.5.1185. PMID: 17490952.
Makris G, Agorastos A, Chrousos GP, Pervanidou P. Stress System Activation in Children and Adolescents With Autism Spectrum Disorder. Front Neurosci. 2022 Jan 13;15:756628. doi: 10.3389/fnins.2021.756628. PMID: 35095389; PMCID: PMC8793840.
Mallorquí-Bagué N, Garfinkel SN, Engels M, Eccles JA, Pailhez G, Bulbena A, Critchley HD. Neuroimaging and psychophysiological investigation of the link between anxiety, enhanced affective reactivity and interoception in people with joint hypermobility. Front Psychol. 2014 Oct 14;5:1162. doi: 10.3389/fpsyg.2014.01162. PMID: 25352818; PMCID: PMC4196473
McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014 May;133(5):872-83. doi: 10.1542/peds.2013-3995. PMID: 24777214.
McGinnis WR, Audhya T, Walsh WJ, Jackson JA, McLaren-Howard J, Lewis A, Lauda PH, Bibus DM, Jurnak F, Lietha R, Hoffer A. Discerning the Mauve Factor, Part 1. Altern Ther Health Med. 2008 Mar-Apr;14(2):40-50. Erratum in: Altern Ther Health Med. 2008 May-Jun;14(3):15. PMID: 18383989.
McGinnis WR, Audhya T, Walsh WJ, Jackson JA, McLaren-Howard J, Lewis A, Lauda PH, Bibus DM, Jurnak F, Lietha R, Hoffer A. Discerning the Mauve factor, Part 2. Altern Ther Health Med. 2008 May-Jun;14(3):56-62. PMID: 18517107.
Monzio Compagnoni G, Di Fonzo A, Corti S, Comi GP, Bresolin N, Masliah E. The Role of Mitochondria in Neurodegenerative Diseases: the Lesson from Alzheimer's Disease and Parkinson's Disease. Mol Neurobiol. 2020 Jul;57(7):2959-2980. doi: 10.1007/s12035-020-01926-1. Epub 2020 May 22. PMID: 32445085; PMCID: PMC9047992.
Morris G, Berk M, Walder K, Maes M. The Putative Role of Viruses, Bacteria, and Chronic Fungal Biotoxin Exposure in the Genesis of Intractable Fatigue Accompanied by Cognitive and Physical Disability. Mol Neurobiol. 2016 May;53(4):2550-71. doi: 10.1007/s12035-015-9262-7. Epub 2015 Jun 17. PMID: 26081141.
Nakanishi G, Pita-Oliveira M, Bertagnolli LS, Torres-Loureiro S, Scudeler MM, Cirino HS, Chaves ML, Miwa B, Rodrigues-Soares F. Worldwide Systematic Review of GSTM1 and GSTT1 Null Genotypes by Continent, Ethnicity, and Therapeutic Area. OMICS. 2022 Oct;26(10):528-541. doi: 10.1089/omi.2022.0090. Epub 2022 Sep 16. PMID: 36112350.
Peters SL, Yao CK, Philpott H, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2016 Sep;44(5):447-59. doi: 10.1111/apt.13706. Epub 2016 Jul 11. PMID: 27397586.
Poznyak AV, Ivanova EA, Sobenin IA, Yet SF, Orekhov AN. The Role of Mitochondria in Cardiovascular Diseases. Biology (Basel). 2020 Jun 25;9(6):137. doi: 10.3390/biology9060137. PMID: 32630516; PMCID: PMC7344641.
Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, Scheibenbogen C, Murovska M, Prusty BK; European Network on ME/CFS (EUROMENE). Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. 2018 Oct 1;16(1):268. doi: 10.1186/s12967-018-1644-y. PMID: 30285773; PMCID: PMC6167797.
Rauch SAM, King A, Kim HM, Powell C, Rajaram N, Venners M, Simon NM, Hamner M, Liberzon I. Cortisol awakening response in PTSD treatment: Predictor or mechanism of change. Psychoneuroendocrinology. 2020 Aug;118:104714. doi: 10.1016/j.psyneuen.2020.104714. Epub 2020 May 15. PMID: 32446108; PMCID: PMC7984524.
Reynolds, A. M., Soke, G. N., Sabourin, K. R., Hepburn, S., Katz, T., Wiggins, L. D., Schieve, L. A., & Levy, S. E. (2019). Sleep Problems in 2- to 5-Year-Olds With Autism Spectrum Disorder and Other Developmental Delays. Pediatrics, 143(3), e20180492. https://doi.org/10.1542/peds.2018-0492
Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. The Heritability of Autism Spectrum Disorder. JAMA. 2017 Sep 26;318(12):1182-1184. doi: 10.1001/jama.2017.12141. PMID: 28973605; PMCID: PMC5818813.
Sharp HEC, Critchley HD, Eccles JA. Connecting brain and body: Transdiagnostic relevance of connective tissue variants to neuropsychiatric symptom expression. World J Psychiatry. 2021 Oct 19;11(10):805-820. doi: 10.5498/wjp.v11.i10.805. PMID: 34733643; PMCID: PMC8546774.
Schmidt R, Hiemisch A, Kiess W, von Klitzing K, Schlensog-Schuster F, Hilbert A. Macro- and Micronutrient Intake in Children with Avoidant/Restrictive Food Intake Disorder. Nutrients. 2021 Jan 27;13(2):400. doi: 10.3390/nu13020400. PMID: 33513954; PMCID: PMC7911718.
Shoemaker RC, House D, Ryan JC. Structural brain abnormalities in patients with inflammatory illness acquired following exposure to water-damaged buildings: a volumetric MRI study using NeuroQuant®. Neurotoxicol Teratol. 2014 Sep-Oct;45:18-26. doi: 10.1016/j.ntt.2014.06.004. Epub 2014 Jun 17. PMID: 24946038.
Sotzny F, Blanco J, Capelli E, Castro-Marrero J, Steiner S, Murovska M, Scheibenbogen C; European Network on ME/CFS (EUROMENE). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome - Evidence for an autoimmune disease. Autoimmun Rev. 2018 Jun;17(6):601-609. doi: 10.1016/ j.autrev.2018.01.009. Epub 2018 Apr 7. PMID: 29635081.
Springer A, Oleksa-Marewska K, Basińska-Zych A, Werner I, Białowąs S. Occupational burnout and chronic fatigue in the work of academic teachers-moderating role of selected health behaviours. PLoS One. 2023 Jan 26;18(1):e0280080. doi: 10.1371/journal.pone.0280080. PMID: 36701360; PMCID: PMC9879519.
Szyf M. The epigenetics of early life adversity and trauma inheritance: an interview with Moshe Szyf. Epigenomics. 2022 Mar;14(6):309-314. doi: 10.2217/epi-2021-0483. Epub 2021 Dec 8. PMID: 34877868.
Theoharides TC, Kavalioti M, Tsilioni I. Mast Cells, Stress, Fear and Autism Spectrum Disorder. Int J Mol Sci. 2019 Jul 24;20(15):3611. doi: 10.3390/ijms20153611. PMID: 31344805; PMCID: PMC6696098.
Tomas C, Newton J, Watson S. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neurosci. 2013 Sep 30;2013:784520. doi: 10.1155/2013/784520. PMID: 24959566; PMCID: PMC4045534.
Traianos E, Dibnah B, Lendrem D, et alAB0051 THE EFFECTS OF NON-INVASIVE VAGUS NERVE STIMULATION ON IMMUNOLOGICAL RESPONSES AND PATIENT REPORTED OUTCOME MEASURES OF FATIGUE IN PATIENTS WITH CHRONIC FATIGUE SYNDROME, FIBROMYALGIA, AND RHEUMATOID ARTHRITISAnnals of the Rheumatic Diseases 2021;80:1057-1058.
Tsai SY, Chen HJ, Lio CF, Kuo CF, Kao AC, Wang WS, Yao WC, Chen C, Yang TY. Increased risk of chronic fatigue syndrome in patients with inflammatory bowel disease: a population-based retrospective cohort study. J Transl Med. 2019 Feb 22;17(1):55. doi: 10.1186/s12967-019-1797-3. PMID: 30795765; PMCID: PMC6387539.
Tsilioni I, Patel AB, Pantazopoulos H, Berretta S, Conti P, Leeman SE, Theoharides TC. IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21659-21665. doi: 10.1073/pnas.1906817116. Epub 2019 Oct 7. PMID: 31591201; PMCID: PMC6815178.
van Rensburg R, Meyer HP, Hitchcock SA, Schuler CE. Screening for Adult ADHD in Patients with Fibromyalgia Syndrome. Pain Med. 2018 Sep 1;19(9):1825-1831. doi: 10.1093/pm/pnx275. PMID: 29099955.
Wang SX, Wu WC. Effects of psychological stress on small intestinal motility and bacteria and mucosa in mice. World J Gastroenterol. 2005 Apr 7;11(13):2016-21. doi: 10.3748/wjg.v11.i13.2016. PMID: 15800998; PMCID: PMC4305729.
Wang T, Yin J, Miller AH, Xiao C. A systematic review of the association between fatigue and genetic polymorphisms. Brain Behav Immun. 2017 May;62:230-244. doi: 10.1016/j.bbi.2017.01.007. Epub 2017 Jan 12. PMID: 28089639; PMCID: PMC5947855.
Weiss RB, Stange JP, Boland EM, Black SK, LaBelle DR, Abramson LY, Alloy LB. Kindling of life stress in bipolar disorder: comparison of sensitization and autonomy models. J Abnorm Psychol. 2015 Feb;124(1):4-16. doi: 10.1037/abn0000014. PMID: 25688428; PMCID: PMC4332547.
Wojcik DP, Godfrey ME, Christie D, Haley BE. Mercury toxicity presenting as chronic fatigue, memory impairment and depression: diagnosis, treatment, susceptibility, and outcomes in a New Zealand general practice setting (1994-2006). Neuro Endocrinol Lett. 2006 Aug;27(4):415-23. PMID: 16891999.
Yang EV, Webster Marketon JI, Chen M, Lo KW, Kim SJ, Glaser R. Glucocorticoids activate Epstein Barr virus lytic replication through the upregulation of immediate early BZLF1 gene expression. Brain Behav Immun. 2010 Oct;24(7):1089-96. doi: 10.1016/j.bbi.2010.04.013. Epub 2010 May 11. PMID: 20466055; PMCID: PMC2939213.
Yap CX, Henders AK, Alvares GA, Wood DLA, Krause L, Tyson GW, Restuadi R, Wallace L, McLaren T, Hansell NK, Cleary D, Grove R, Hafekost C, Harun A, Holdsworth H, Jellett R, Khan F, Lawson LP, Leslie J, Frenk ML, Masi A, Mathew NE, Muniandy M, Nothard M, Miller JL, Nunn L, Holtmann G, Strike LT, de Zubicaray GI, Thompson PM, McMahon KL, Wright MJ, Visscher PM, Dawson PA, Dissanayake C, Eapen V, Heussler HS, McRae AF, Whitehouse AJO, Wray NR, Gratten J. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell. 2021 Nov 24;184(24):5916-5931.e17. doi: 10.1016/j.cell.2021.10.015. Epub 2021 Nov 11. PMID: 34767757.
Yuzefovych LV, Pastukh VM, Ruchko MV, Simmons JD, Richards WO, Rachek LI. Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS One. 2019 Oct 10;14(10):e0222278. doi: 10.1371/journal.pone.0222278. PMID: 31600210; PMCID: PMC6786592.